NIH scientists develop faster, less costly COVID-19 test
National Institute of Health scientists have developed a new sample preparation method to detect SARS-CoV-2, the virus behind COVID-19.
The new method bypasses extraction of COVID-19’s genetic RNA material, something that current COVID-19 tests do, and goes straight to simplifying sample purification. The new process may reduce test time and cost, according to the NIH.
Standard testing for SARS-CoV-2 virus amplifies viral RNA to detectable levels using a technique called quantitative reverse transcription PCR. Before the amplification efforts begin, the RNA must be extracted from the sample.
RELATED: Delta, American Airlines, Southwest will not mandate COVID-19 vaccine for employees
RNA extraction kit manufacturers have had difficulty keeping up with demand during the COVID-19 pandemic, hindering testing capacity worldwide.
The NIH said that with new coronavirus variants emerging, the need for better and faster testing is greater than ever.
Dr. Robert B. Hufnagel, chief of the NEI Medical Genetics and Ophthalmic Genomic Unit, and Bin Guan, Ph.D., a fellow at the Ophthalmic Genomics Laboratory at NEI, made the discovery for the new test.
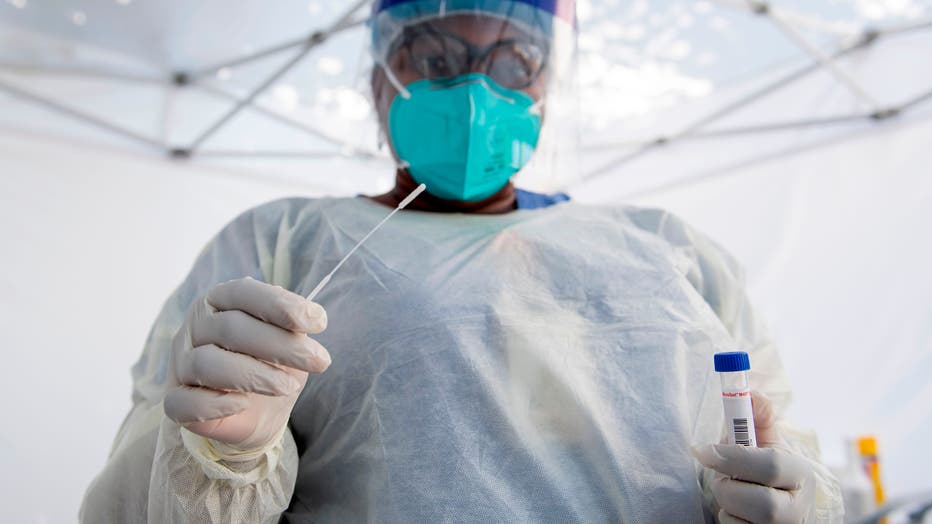
A health worker takes a nasal swab sample at a COVID-19 testing site at St. John's Well Child and Family Center, amid the novel coronavirus pandemic, July 24, 2020, in Los Angeles, California. (Photo by VALERIE MACON/AFP via Getty Images)
Hufnagel’s team tested a variety of chemicals on synthetic and human samples to identify those that could preserve RNA in samples with minimal degradation while still allowing direct detection of the virus.
"We used nasopharyngeal and saliva samples with various virion concentrations to evaluate whether they could be used for direct RNA detection," said Guan, the lead author of a report on the technique, which was published this week in iScience. "The answer was yes, with markedly high sensitivity. Also, this preparation inactivated the virus, making it safer for lab personnel to handle positive samples."
RELATED: COVID-19 Delta variant symptoms: What we know and what to look for
In a test to validate the new discoveries, the NIH found that the new preparation technique significantly increased the RNA yield available for testing compared to the standard method.
"We think this novel methodology has clear benefits of increasing sensitivity, cost and time savings for testing," said Hufnagel, "The method stabilizes the RNA at room temperature for easier transport, storage, and handling in clinical settings."
This story was reported from Los Angeles.

